Why bother to know soil pH? Does soil pH really matter to your garden plants? You garden just to have fun. Bring up soil pH seems serious. But do you know that the wrong soil pH has caused you to lose quite a lot of money, time and energy?
Soil pH falls out of neutral will cause some nutrients to be fixed, thus plants can’t absorb them. The most suitable soil pH for nutrient availability is 6.5~7.5. If it falls out of this range, the fertilizer you put in the soil will be useless. It’s a waste of money (buying the fertilizer), time (trips you made to the garden center, applying the fertilizer ) and energy (production of fertilizer).
1. Soil pH causes phosphorus unavailable to plants
This chart tells us what happens to nutrient availability with soil pH variation:

|
| Source: University of Florida Agriculture Extension |
Among all nutrients, phosphorus has the tightest range for uptake. When soil pH is below 6.0, phosphorus will be in the form of HPO4-2, fixed with cations of iron or aluminum and not available to plants. If pH is above 7.5, more phosphorus will become fixed with calcium, making it unavailable to plants, either.
How to know if plants are lacking in phosphorus?
Plants lacking in phosphorus often show small leaves and unusual blue-green, reddish-violet or pale in severe deficiencies. The violet color develops from increased anthocyanin synthesis.
Phosphorus is essential to plant life. Plants that lack phosphorus are stunted in growth because phosphorus is a component of nucleic acid, protein and energy transformation. Plants simply can’t survive with a shortage of phosphorus.
2. Soil pH affects soil nitrogen
Nitrogen absorption seems to work within a fairly big range of pH from 6.0~8.5, but things are usually not as simple as they seem.
A pH lower than 7 has negative effects on nitrogen availability. Most garden plants take nitrate. Nitrate dissolves in the water. If plants don't take them, they will leach quickly. While ammonium attaches to the soil, they need the nitrifying bacteria to convert them into nitrate. The nitrifying bacteria are very sensitive to soil pH.
There are two nitrifying bacteria, nitrosomonas and nitrobacter. The nitrosomonas has an optimal pH between 7.0~8.0. The nitrobacter is 7.5~8.0. When soil pH falls out of these optimal ranges, the activity of nitrifying bacteria will drop sharply (Nitrification: United States Environmental Protection Agency, 2002).
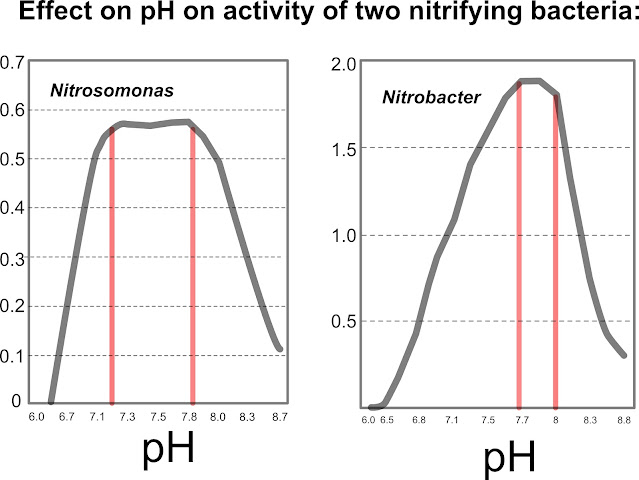
|
|
Source: Grady, C.P.L, Jr., and H.C. Lim, 1980. Biological Wastewater Treatment. Marcel Dekker, NY |
However, when nitrifying bacteria convert ammonium to nitrate, it will release 3 H+ to the soil, which lowers the pH.
A slightly high pH causes the loss of ammonium from the soil. When soil pH gets above 8, 10% of the ammonium becomes ammonia(gas) and evaporates out of the soil.
If you use synthetic fertilizer, maintaining soil pH in a neutral range (7.0~8.0) is crucial to make your fertilizer work. So you won’t waste your money, time and energy. Unfortunately, the synthetic chemical accumulates overtime and changes the soil quality. The soil will fail to buffer the pH.
3. Adding Compost brings soil pH to neutral
The easiest and cheapest way to restore the buffer of soil pH is by adding the compost. In the field, we deal more acid than alkaline soil. The alkaline soil is often from irrigation or parent material. However, compost can buffer the soil pH by absorbing cations because compost is negatively charged. Because of its high CEC, compost prevents soil pH from getting low by absorbing H+ accumulated over time. The H+ is what causes the low soil pH.

|
| Compost holds the H+ in the soil so it won't get into the soil water and lower the soil pH |
Compost helps alkaline soil to be closer to neutral too. There is still some organic matter in the compost. When soil microbes decompose the organic matter, it will release CO2 and then forms carbonic acid. Organic acids are also released during humus decomposition, which helps lower the soil pH.





0 Comments